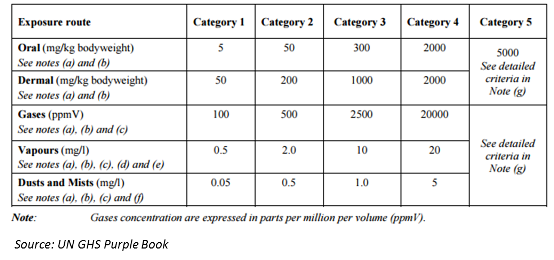
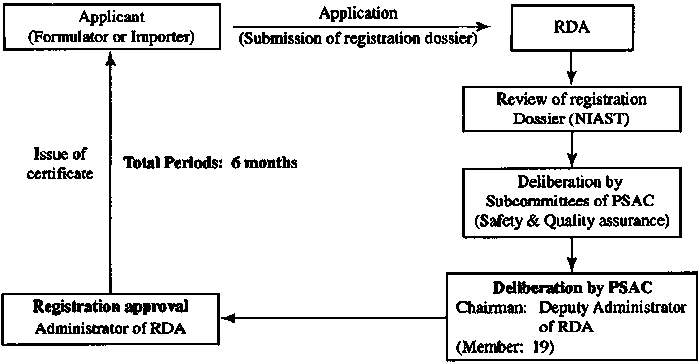
Pesticide Management in India
1. Pesticide regulation 1) 《The Insecticides Act, 1968》; 2)《Insecticides Rules,1971》
2. Pesticide administration department
Name |
Function |
The Central Insecticides Board |
The committee advises the central government and state governments on the implementation of the relevant technical issues and authorizes the implementation of the law. |
Registration Committee |
Review pesticide registration applications submitted by importers and producers and then register them;
Implementing other functions granted by The Pesticide Act, 1968. |
Central Insecticide Laboratory |
Authorized by the Central Government or the Agricultural Sector to verify the properties of all pesticide products in India. |
3. Registration Types and Material Requirements
Registration Types Registration of new compounds 9(3); Temporary registration of new compounds 9(3b); Registration of same products (preparations) 9(4).
Registration procedure For the registration of pesticides in India, the first step is to apply for import license for sample testing. After entering India, the samples were analyzed by the Central Insecticide Laboratory for physical and chemical properties, product quality, field efficacy and storage stability at room temperature. Based on this analysis and other data provided by the applicant, the product registration application can be submitted to the Central Insecticides Board of India.
Material Requirements 1) Legal documents A letter of authorization from the factory to India registration company; Production license of factory; A legal inspection report on the first shipment of goods to India.
2) Chemical Characters Product technical specifications; Physicochemical properties report; Quality analysis report; Single batch full analysis report; Storage report at room temperature for 2 years.
3) Toxicology Acute toxicity report; Mouse oral, rat oral, rabbit skin, rabbit skin stimulation, rabbit mucosa stimulation, rat inhalation; Mouse, rat, rabbit skin, rabbit skin, rabbit mucosa, rat inhalation; Rat and dog subchronic oral toxicity test; Rat subchronic percutaneous toxicity test; Rat subchronic inhalation test; Trigenic test; Environmental toxicity test.
4) Biology Metabolism of the original drug in soil, water and plants; Persistence report of the original drug in soil, water and plant; Pharmacodynamic experiment; Plant toxicity experiment; Residue experiment.
5) Package and label According to Indian regulations, the label should include product percentage composition, product purpose, antidotes, toxicity labeling, precautions and use restrictions. The brochure should include product introduction, toxicity and rescue measures, storage or abandonment precautions, and detailed packaging information.
6) Official registration certificate must be provided.
4. Registration approval time
Item |
9(4)-- Registration of Same Product |
9(3)and 9(3b)--
New Product Registration |
Fill in Form C |
Half a month |
1 month |
Mail and analyze samples |
2-6 months |
6 months |
Official experimental analysis
Chemical Character Analysis
Pharmacodynamic experiment
Toxicological test
Package and Storage Tests |
1-3 months |
6-24 months, the storage test takes 2 years. |
Determining MRL |
1-2 months |
6-12 months |
Issuance of Registration Certificate |
2 months |
2 months |
Total |
At least 6 months |
12-36 months |
|
|