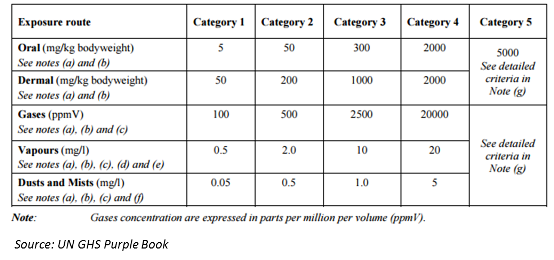
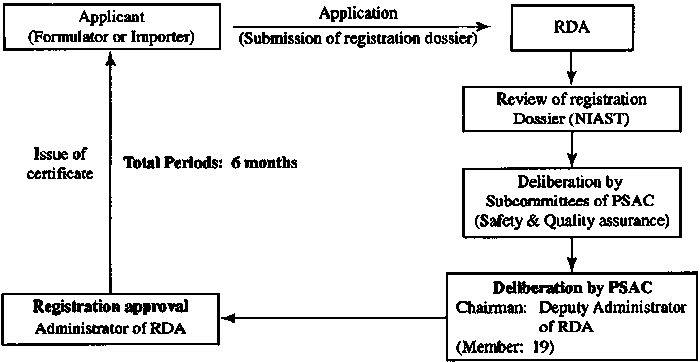
Pesticide Management in Thailand
1. Pesticide regulation 《The Hazardous Substances Act》
2. Pesticide administration department
Pesticide is carried out by the Toxic Substances Division of the Agricultural Department in Thailand. There are four sections in the Toxic Substances Division of the Agricultural Department: 1) The Pesticide Registration Section is responsible for the registration and management of pesticides throughout the country; 2) The Residue Section is responsible for pesticide residue detection in the environment after application; 3) The Preparation Section is responsible for the quality inspection of pesticides in the circulation field, the development, research and management of pesticide preparations; 4) Pesticide toxicity office is responsible for the study of human hazards in pesticide application. Knowing the safe use of drugs.
3. Registration Types and Material Requirements
Registration Types The license issued by the Ministry of Agriculture contains the following categories: 1) Production license; 2) Import license; 3) Ownership (including sales, storage, transportation and service) license.
Registration procedure Phase one: trials clearance; Phase two: provisional clearance;
Phase three: full registration.
Material Requirements
1) Document requirements of phase one
Free sale certificate of preparations; Six acute toxicity reports of original drugs and preparations; General description of physicochemical properties of the original drug; General description of preparation traits; Summarize of environmental toxicity; Analysis methods of raw drugs and preparations, etc.
2) Document requirements of phase two
The main task of this phase is to carry out the efficacy and residue tests in Thailand for 2 years and 2 places. Generally, no additional data is required, but the following information may be added according to the requirements of the Ministry of Agriculture of Thailand: Acute toxicity test data; Chronic toxicity test data; Trigenic test data; Neurotoxicity data; Residual test data.
2) Document requirements of phase three
The following materials need to be added: Field test results and residual test results; If the community demonstration test is carried out in the phase two, it can also be submitted; Complete physicochemical properties of the preparation; Complete toxicity data of the original drug and preparation; Residue analysis method; MRL information in the environment; Packaging information; Label of local text; MSDS.
4. Registration approval time
Number |
Registration Type |
Time |
1 |
Phase one |
6-12 months |
2 |
Phase two |
12 months |
3 |
Phase three |
6-12 months |
|