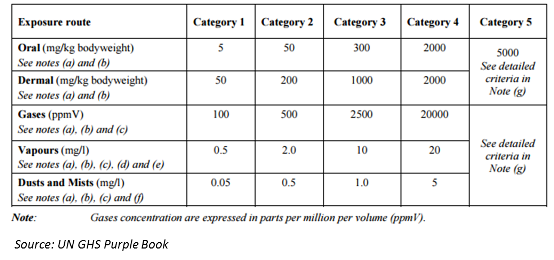
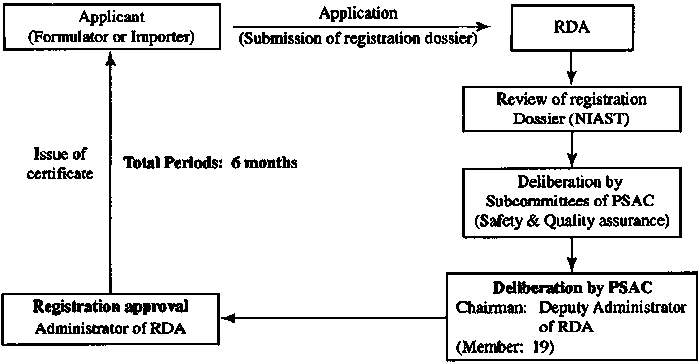
Pesticide Management in Indonesia
1. Pesticide regulation The registration and licensing system of pesticides was started in the 1970s. Its legal basis is No. 7 of the Government Act 1973.
2. Pesticide administration department The Pesticide Committee of the Ministry of Agriculture is responsible for pesticide management. The pesticide Association assists the pesticide Committee of the Ministry of Agriculture in the management of pesticides in terms of laws and ethics.
3. Registration Types and Material Requirements
Registration Types For prohibited pesticides, the registration system shall be implemented, and three registration certificates shall be issued, namely, test registration certificate, temporary registration certificate and official registration certificate.
Material Requirements Material requirements are divided into eight parts: product chemistry, scope and method of use, pharmacodynamics and toxicity, toxicology, residue, environmental toxicity, producer information, and other information.
The product chemistry includes: The name of pesticide preparations; Pesticide categories; Preparations categories; Product specifications and physicochemical properties of preparations; Composition of preparations; Compatibility of preparations with other pesticides; The name and chemical formula of active ingredients; Physical properties of active ingredients; Composition of original drugs; Preparation analysis methods; Residue analysis methods; The duration of validity in the environment, etc.
The scope and method of use includes: Use range; Recommended dose or concentration; Application time; Application method; Safety interval, etc.
The pharmacodynamics and toxicity includes: Target insect activity mode; Efficacy test results; Distribution of residual pests after use; Sensitive crops; Elimination time of pesticide damage, etc.
The toxicology includes: Acute toxicity, Subchronic and chronic toxicity(Skin and eye irritation, Sensitization);Medical data, etc.
The residue includes: Residue determination; Dosage; Interval time; Safety interval; Sampling time; Analysis time; Residue value and data source, etc.
The environmental toxicity includes: Laboratory data on acute toxicity of fish; Field data on toxicity of fish; EC50 or LC50 data of local aquatic organisms; Transformation data of pesticides in abiotic environment; Cumulative data of pesticides in abiotic and biological environment; Toxicity information of pesticides to wildlife and environment; Metabolism of Pesticides in Animals and Plants; Persistence and leaching of pesticides in water and soil.
Other information includes: Disposal methods of waste pesticides; Packaging containers; Labels; Registration and revocation in other countries.
4. Registration approval time
The registration information provided by the enterprise shall be submitted by the Indonesian partner to the Ministry of Agriculture of Indonesia. After assessment and confirmation (up to 30 days), the qualified person may issue a test registration certificate.
Pharmacodynamic and toxicological tests were carried out after the quality test of laboratory samples was qualified.
After the quality of laboratory samples is qualified, pharmacodynamic and toxicological tests were carried out. After the test report is approved by the pesticide committee, a temporary registration certificate or a formal registration certificate will be issued. The whole process will take about one year. If the pharmacodynamic and toxicological report of the product has been done in China, the whole registration can be completed within 9 months. |
|